The Centers for Disease Control and Prevention (CDC) V-safe website has stopped reporting on complaints of adverse events pertaining to Covid vaccines without any reason or explanation.
According to the V-safe website’s statement: “Thank you for your participation. Data collection for COVID-19 vaccines concluded on June 30, 2023.” If you go there today, V-safe directs users to the FDA’s VAERS website for adverse event reporting, even though officials continually derided VAERS as “passive” and “unverified.”
The FDA and CDC, respectively, run the mutually exclusive safety collection databases VAERS and V-safe. While V-safe is a device “app” that requires online registration, VAERS is an earlier method of gathering safety data that allows users to complete a form manually, online, or by phoning a toll-free number. While V-safe was an active collecting system targeted at a younger app-using audience, VAERS was a passive collection system. Both systems capture personal information, lot numbers, dates, and associated information.
Here is the last report before deletion.
The problem with the stoppage
Compare that to the National Highway Traffic and Safety Administration’s (NHTSA) continued acceptance of a safety report for a Ford Bronco II that is 30 years old.
Similar to mRNA shots, Bronco IIs are still on the market, and individuals continue to use them today. It doesn’t quite make sense why a 30 year old car would continue to report on possible defects, but a vaccine which was just developed, would not.
NHTSA is still accepting safety reports on things like a 30-year-old Ford Bronco II, but the CDC isn’t accepting new safety reports on 2-year old novel mRNA vaccines.
CDC No longer accepting safety reports despite rapidly increasing safety findings:
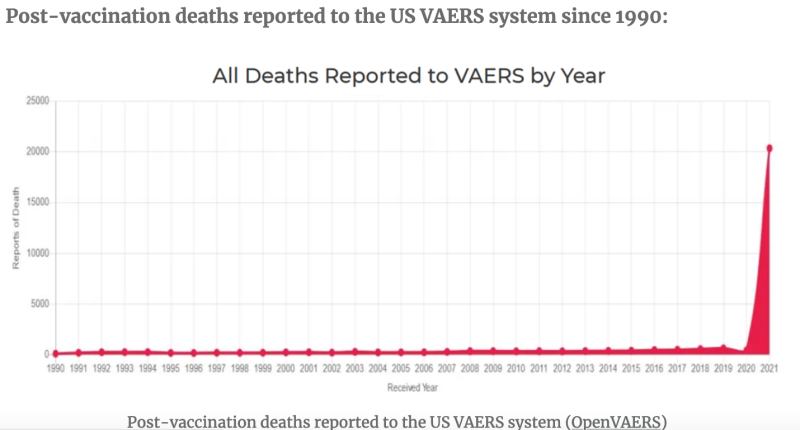
The FDA Vaccine Adverse Event Reporting System (VAERS) database states that mRNA “vaccines” have been named the primary suspect in over 1.5 million adverse event reports
, including >
20,000 heart attacks and >
27,000 cases of myocarditis and pericarditis just in the USA alone. In contrast to an old Bronco, mRNA injections have only been available for about two years.There would be more people in the world. Numerous sources, including a Harvard study that was supported by the FDA, claim that true vaccination adverse events account for
less than 1% of VAERS reports.
On a Ford Bronco II, the NHTSA website only lists one parts recall, one investigation, and 23 complaints, but it still has a button in the upper right corner for reporting new complaints.
“Singular event or a series of events that are threatening in terms of health, safety or well-being of a community or large group of people.” is how Wikipedia defines a humanitarian crisis or humanitarian disaster. Adverse occurrences from mRNA shots in the USA alone could be regarded as a humanitarian catastrophe based on VAERS and prior V-safe findings.
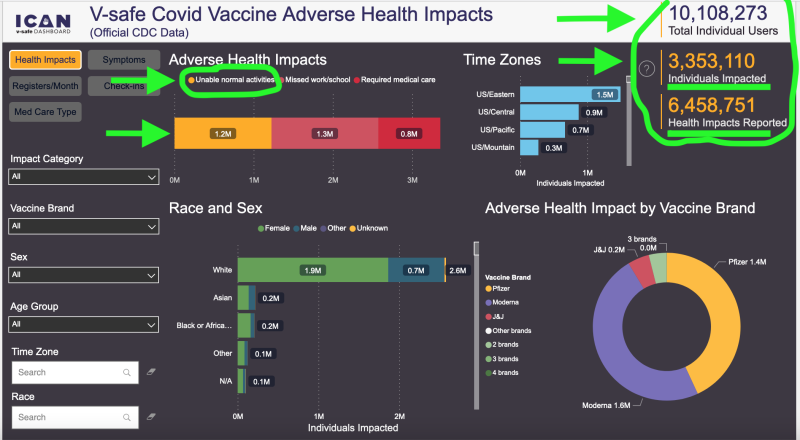
Despite those concerning clinical findings, the CDC has come to the conclusion that it is no longer in the national interest to collect additional safety reports. Out of 10.1 million users, the V-safe website’s statistics indicated approximately 6.5 million negative adverse events/health reactions, with almost 2 million of those being unable to carry out regular daily activities or requiring medical attention. In other words, it’s “case closed” with regard to gathering new safety reports under the current federal public health administration, despite the fact that mRNA shots are still readily accessible and that the CDC encourages their ongoing use.


You must be logged in to post a comment Login