Big Pharma
Big Pharma is now the Leading Cause of Death in America
Published
2 years agoon

Overtreatment with drugs kills many people, and the death rate is increasing. It is therefore strange that we have allowed this long-lasting drug pandemic to continue, and even more so because most of the drug deaths are easily preventable.
In 2013, I estimated that our prescription drugs are the third leading cause of death after heart disease and cancer,1 and in 2015, that psychiatric drugs alone are also the third leading cause of death.2 However, in the US, it is commonly stated that our drugs are “only” the fourth leading cause of death.3,4 This estimate was derived from a 1998 meta-analysis of 39 US studies where monitors recorded all adverse drug reactions that occurred while the patients were in hospital, or which were the reason for hospital admission.
This methodology clearly underestimates drug deaths. Most people who are killed by their drugs die outside hospitals, and the time people spent in hospitals was only 11 days on average in the meta-analysis.5 Moreover, the meta-analysis only included patients who died from drugs that were properly prescribed, not those who died as a result of errors in drug administration, noncompliance, overdose, or drug abuse, and not deaths where the adverse drug reaction was only possible.
Many people die because of errors, e.g. simultaneous use of contraindicated drugs, and many possible drug deaths are real. Moreover, most of the included studies are very old, the median publication year being 1973, and drug deaths have increased dramatically over the last 50 years. As an example, 37,309 drug deaths were reported to the FDA in 2006 and 123,927 ten years later, which is 3.3 times as many.
In hospital records and coroners’ reports, deaths linked to prescription drugs are often considered to be from natural or unknown causes. This misconception is particularly common for deaths caused by psychiatric drugs. Even when young patients with schizophrenia suddenly drop dead, it is called a natural death. But it is not natural to die young and it is well known that neuroleptics can cause lethal heart arrhythmias.
Many people die from the drugs they take without raising any suspicion that it could be an adverse drug effect. Depression drugs kill many people, mainly among the elderly, because they can cause orthostatic hypotension, sedation, confusion, and dizziness. The drugs double the risk of falls and hip fractures in a dose-dependent manner, and within one year after a hip fracture, about one-fifth of the patients will have died. As elderly people often fall anyway, it is not possible to know if such deaths are drug deaths.
Another example of unrecognised drug deaths is provided by non-steroidal anti-inflammatory drugs (NSAIDs). They have killed hundreds of thousands of people, mainly through heart attacks and bleeding stomach ulcers, but these deaths are unlikely to be coded as adverse drug reactions, as such deaths also occur in patients who do not take the drugs.
The 1998 US meta-analysis estimated that 106,000 patients die every year in hospital because of adverse drug effects (a 0.32% death rate). A carefully done Norwegian study examined 732 deaths that occurred in a two-year period ending in 1995 at a department of internal medicine, and it found that there were 9.5 drug deaths per 1,000 patients (a 1% death rate). This is a much more reliable estimate, as drug deaths have increased markedly. If we apply this estimate to the US, we get 315,000 annual drug deaths in hospitals. A review of four newer studies, from 2008 to 2011, estimated that there were over 400,000 drug deaths in US hospitals.
Drug usage is now so common that newborns in 2019 could be expected to take prescription drugs for roughly half their lives in the US.12 Moreover, polypharmacy has been increasing.
How Many People Are Killed by Psychiatric Drugs?
If we want to estimate the death toll of psychiatric drugs, the most reliable evidence we have are the placebo-controlled randomised trials. But we need to consider their limitations.
First, they usually run for only a few weeks even though most patients take the drugs for many years.
Second, polypharmacy is common in psychiatry, and this increases the risk of dying. As an example, the Danish Board of Health has warned that adding a benzodiazepine to a neuroleptic increases mortality by 50-65%.
Third, half of all deaths are missing in published trial reports. For dementia, published data show that for every 100 people treated with a newer neuroleptic for ten weeks, one patient is killed. This is an extremely high death rate for a drug, but FDA data on the same trials show it is twice as high, namely two patients killed per 100 after ten weeks. And if we extend the observation period, the death toll becomes even higher. A Finnish study of 70,718 community-dwellers newly diagnosed with Alzheimer’s disease reported that neuroleptics kill 4-5 people per 100 annually compared to patients who were not treated.
Fourth, the design of psychiatric drug trials is biased. In almost all cases, patients were already in treatment before they entered the trial, and some of those randomised to placebo will therefore experience withdrawal effects that will increase their risk of dying, e.g. because of akathisia. It is not possible to use the placebo-controlled trials in schizophrenia to estimate the effect of neuroleptics on mortality because of the drug withdrawal design. The suicide rate in these unethical trials was 2-5 times higher than the norm.20 One in every 145 patients who entered the trials of risperidone, olanzapine, quetiapine, and sertindole died, but none of these deaths were mentioned in the scientific literature, and the FDA didn’t
require them to be mentioned.
Fifth, events after the trial is stopped are ignored. In Pfizer’s trial of sertraline in adults, the risk ratio for suicides and suicide attempts was 0.52 when the follow-up was only 24 hours, but 1.47 when the follow-up was 30 days, i.e. an increase in suicidal events. And when researchers reanalysed the FDA trial data on depression drugs and included harms occurring during followup, they found that the drugs double the number of suicides in adults compared to placebo.
In 2013, I estimated that, in people aged 65 and above, neuroleptics, benzodiazepines, or similar, and depression drugs kill 209,000 people annually in the United States. I used rather conservative estimates, however, and usage data from Denmark, which are far lower than those in the US. I have therefore updated the analysis based on US usage data, again focusing on older age groups.
For neuroleptics, I used the estimate of 2% mortality from the FDA data.18
For benzodiazepines and similar drugs, a matched cohort study showed that the drugs doubled the death rate, although the average age of the patients was only 55.25 The excess death rate was about 1% per year. In another large, matched cohort study, the appendix to the study report shows that hypnotics quadrupled the death rate (hazard ratio 4.5). These authors estimated that sleeping pills kill between 320,000 and 507,000 Americans every year.26 A reasonable estimate of the annual death rate would therefore be 2%.
For SSRIs, a UK cohort study of 60,746 depressed patients older than 65 showed that they led to falls and that the drugs kill 3.6% of patients treated for one year.27
Another cohort study, of 136,293 American postmenopausal women (age 50-79) participating in the Women’s Health Initiative study, found that depression drugs were associated with a 32% increase in all-cause mortality after adjustment for confounding factors, which corresponded to 0.5% of women killed by SSRIs when treated for one year.28 The death rate was very likely underestimated. The authors warned that their results should be interpreted with great caution, as the way exposure to antidepressant drugs was ascertained carried a high risk of misclassification, which would make it more difficult to find an increase in mortality. Further, the patients were much younger than in the UK study, and the death rate increased markedly with age and was 1.4% for those aged 70-79. Finally, the exposed and unexposed women were different for many important risk factors for early death, whereas the people in the UK cohort were their own control.
For these reasons, I decided to use the average of the two estimates, a 2% annual death rate.
These are my results for the US for these three drug groups for people at least 65 years of age (58.2 million; usage is in outpatients only):29-32
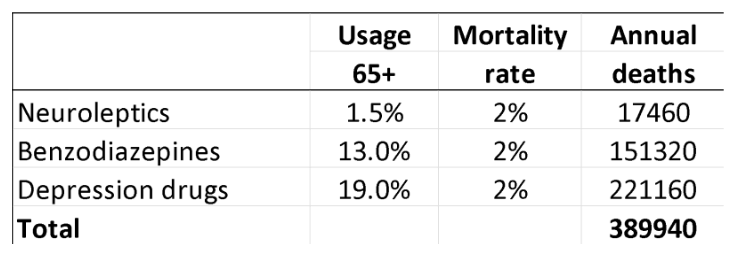
A limitation in these estimates is that you can only die once, and many people receive polypharmacy. It is not clear how we should adjust for this. In the UK cohort study of depressed patients, 9% also took neuroleptics, and 24% took hypnotics/anxiolytics.
On the other hand, the data on death rates come from studies where many patients were also on several psychiatric drugs in the comparison group, so this is not likely to be a major limitation considering also that polypharmacy increases mortality beyond what the individual drugs cause.
Statistics from the Centers for Disease Control and Prevention list these four top causes of death: 33
Heart disease: 695,547
Cancer: 605,213
Covid-19: 416,893
Accidents: 224,935
Covid-19 deaths are rapidly declining, and many such deaths are not caused by the virus but merely occurred in people who tested positive for it because the WHO advised that all deaths in people who tested positive should be called Covid deaths.
Young people have a much smaller death risk than the elderly, as they rarely fall and break their hip, which is why I have focused on the elderly. I have tried to be conservative. My estimate misses many drug deaths in those younger than 65 years; it only included three classes of psychiatric drugs; and it did not include hospital deaths.
I therefore do not doubt that psychiatric drugs are the third leading cause of death after heart disease and cancer.
Other Drug Groups and Hospital Deaths
Analgesics are also major killers. In the US, about 70,000 people were killed in 2021 by an overdose of a synthetic opioid.
The usage of NSAIDs is also high. In the US, 26% of adults use them regularly, 16% of which get them without a prescription (mostly ibuprofen and diclofenac).
As there seems to be no major differences between the drugs in their capacity to cause thromboses,37 we may use data for rofecoxib. Merck and Pfizer underreported thrombotic events in their trials of rofecoxib and celecoxib, respectively, to such an extent that it constituted fraud,1 but in one trial, of colorectal adenomas, Merck assessed thrombotic events. There were 1.5 more cases of myocardial infarction, sudden cardiac death or stroke on rofecoxib than on placebo per 100 patients treated.38 About 10% of the thromboses are fatal, but heart attacks are rare in young people. Restricting the analysis to those aged at least 65, we get 87,300 annual deaths.
It has been estimated that 3,700 deaths occur each year in the UK due to peptic ulcer complications in NSAID users,39 corresponding to about 20,000 deaths each year in the US. Thus, the total estimate of NSAID deaths is about 107,000.
If we add the estimates above, 315,000 hospital deaths, 390,000 psychiatric drug deaths, 70,000 synthetic opioid deaths, and 107,000 NSAID deaths, we get 882,000 drug deaths in the United States annually.
Many commonly used drugs other than those mentioned above can cause dizziness and falls, e.g. anticholinergic drugs against urinary incontinence and dementia drugs, which are used by 1% and 0.5% of the Danish population, respectively, even though they do not have any clinically relevant effects.1,2
It is difficult to know what the exact death toll of our drugs is, but there can be no doubt that they are the leading cause of death. And the death toll would be much higher if we included people below 65 years of age. Moreover, from the official number of deaths from heart disease, we would need to subtract those caused by NSAIDs, and from accidents, deaths by falls caused by psychiatric drugs and many other drugs.
If such a hugely lethal pandemic had been caused by a microorganism, we would have done everything we could to get it under control. The tragedy is that we could easily get our drug pandemic under control, but when our politicians act, they usually make matters worse. They have been so heavily lobbied by the drug industry that drug regulation has become much more permissive than it was in the past.40
Most of the drug deaths are preventable,41 above all because most of the patients who died didn’t need the drug that killed them. In placebo-controlled trials, the effect of neuroleptics and depression drugs has been considerably below the least clinically relevant effect, also for very severe depression.2,7 And, despite their name, non-steroidal, anti-inflammatory drugs, NSAIDs do not have anti-inflammatory effects,1,42 and systematic reviews have shown that their analgesic effect is similar to that of paracetamol (acetaminophen). Yet, most patients with pain are recommended to take both paracetamol and an NSAID over the counter. This will not increase the effect, only the risk of dying.
Most tragically, leading psychiatrists all over the world do not realise how ineffective and dangerous their drugs are. A US psychiatrist, Roy Perlis, professor at Harvard, argued in April 2024 that depression pills should be sold over the counter because they are “safe and effective.”43 They are highly unsafe and ineffective. Perlis also claimed that depression drugs do not increase the risk of suicide in people older than 25, which is also wrong. They double suicides in adults.23,24
Perlis wrote, “Some still question the biological basis of this disorder, despite the identification of more than 100 genes that increase depression risk and neuroimaging studies showing differences in the brains of people with depression.” Both of these claims are plain wrong. Genetic association studies have come up empty-handed and so have brain imaging studies, which are generally highly flawed.44 People are depressed because they live depressing lives, not because of some brain disorder.
SOURCE: BROWNSTONE INSTITUTE
You may like
-


New Bill Aims to Remove COVID-19 Vaccine Manufacturer Liability Protections
-


Docs unveiled show Big Pharma used Ukraine as ‘Guinea Pig for Human Testing’
-


Zelensky-Backed Billionaire Arrested For Fraud and Money Laundering
-


Pharmaceutical Firm Linked to 2024 Candidate Ramaswamy Suid For COVID Vaccine Technology
-


Big Pharma Gets Paid By Governments For Vaccines Whether Vials Get Used Or Not
Big Pharma
Abortion Pill Complications 22X Higher Than Previously Reported, Per New Study
Published
9 months agoon
April 28, 2025
A newly released analysis is raising serious questions about the safety profile of mifepristone, the drug responsible for over half of abortions in the United States. While abortion-rights advocates, corporate media outlets, and the U.S. Food and Drug Administration (FDA) maintain that the drug is “safe and effective,” a comprehensive study based on real-world insurance claims paints a far more concerning picture.
Described as the “largest known study of the abortion pill,” the report was conducted by Ethics and Public Policy Center President Ryan Anderson and Director of Data Analysis Jamie Bryan Hall. Using a massive dataset that included Medicaid, TRICARE, Medicare, Department of Veterans Affairs, and private insurance claims, the researchers analyzed 865,727 prescriptions of mifepristone distributed to 692,873 women between 2017 and 2023.
The findings are striking: approximately 10.9 percent of those chemical abortions—about 94,605 cases—involved potentially life-threatening “serious adverse events” within 45 days of taking the drug. These complications included emergency room visits, hemorrhage, sepsis, infection, and follow-up surgeries. This complication rate is at least 22 times higher than the <0.5 percent figure cited by the FDA on the Mifeprex label.
The researchers noted that some patients experienced complications in multiple categories, and that the 45-day window used for measurement was “conservative,” especially considering that the FDA has relied on studies using a timeframe of up to 72 days.
One chart from the study revealed that among women who sought post-abortion care within 45 days:
- 15.1% visited the emergency room,
- 8.5% required surgical treatment,
- 2.5% experienced hemorrhage,
- 1.9% suffered infections, and
- 0.9% were diagnosed with sepsis.
“These outcomes were drawn from actual claims data,” the researchers emphasized, “not modeled projections or self-reported surveys.” In Anderson’s words to The Federalist: “This study is the statistical equivalent of a category 5 hurricane hitting the prevailing narrative of the abortion industry. It reveals, based on real-world data, the shocking number of women who suffer serious medical consequences because of the abortion pill.”
The FDA originally approved mifepristone in 2000 based on 10 clinical trials involving only 30,966 patients—women who were described as “prescreened,” “generally healthy,” and treated in controlled environments. The authors of the new study argue that those trials are both outdated and unrepresentative of today’s broad and diverse patient base.
“The women in our dataset receive (or fail to receive) pre- and post-abortion healthcare of the real-world quality that prevails in the U.S. today, not the carefully controlled regimen of care that ordinarily prevails in a clinical trial,” the study says.
Despite repeated petitions from pro-life medical groups to revisit the approval of mifepristone, the FDA has consistently declined to take action. Critics argue the agency failed to meet its legal obligation to address the concerns. Meanwhile, regulatory oversight has continued to loosen. By 2016, the FDA under the Obama administration had altered the drug’s dosing, cut down the number of in-person doctor visits required, broadened who could prescribe it, and eliminated requirements to report non-fatal complications.
The Biden administration went further. In 2021, the FDA permanently allowed mifepristone to be delivered by mail, bypassing the need for a clinic visit. Pharmacies like Walgreens and CVS were later authorized to dispense the pill. As of 2023, a woman can obtain mifepristone with just one telehealth appointment with “any approved healthcare provider (not necessarily a physician)” and self-administer the drug at home. Alarmingly, prescribers are not required to report adverse events unless they learn the patient has died.
The study recommends that the FDA reinstate its original safety protocols. These would include requiring multiple in-person visits, physician-only prescribing, ultrasound confirmation of gestational age and the absence of ectopic pregnancy, and mandatory reporting of complications. The goal, according to the authors, is not only to reduce immediate harm but also to facilitate better long-term safety tracking.
“The FDA should further investigate the harm this drug causes to women and, based on objective safety criteria, reconsider its approval altogether. Women deserve better than the abortion pill,” the study concludes.
While legal efforts to challenge the pill’s availability have so far been unsuccessful, the issue remains live. In 2023, the Supreme Court declined to weigh in on the merits of mifepristone’s approval, ruling that the plaintiffs lacked standing. However, Justice Brett Kavanaugh’s opinion left open the possibility for the Court to consider a more suitable challenge in the future.
SOURCE: THE FEDERALIST
Big Pharma
Doctor Fails to Publish $10 Million Taxpayer-Funded Study Exposing Puberty Blockers
Published
1 year agoon
October 23, 2024
A $10 million study on the mental health effects of puberty blockers in transgender youth has become a focal point in the ongoing debate over gender-affirming care in the United States. Dr. Johanna Olson-Kennedy, a prominent advocate for transgender rights and the study’s lead researcher, has chosen to withhold the results, citing concerns that the findings could be used against gender-affirming care for children. This decision has sparked criticism from both fellow researchers and opponents of puberty blockers, who argue that withholding the study is contrary to scientific standards.
The National Institutes of Health (NIH)-funded study, which began in 2015, tracked 95 children, with an average starting age of 11, as they received puberty blockers—medications designed to delay the onset of physical changes during puberty. After two years, the study found that the mental health of the children had not improved as a result of the treatment. Olson-Kennedy explained in an interview with The New York Times that the participants’ mental health was relatively stable before and after treatment, attributing the lack of significant change to the children being “in really good shape” mentally from the outset.
However, this finding contradicts earlier data gathered by the study, which indicated that roughly 25% of participants experienced symptoms of depression or suicidal thoughts before starting treatment. The decision not to publish has raised questions about scientific transparency and integrity, particularly in a field where public opinion is sharply divided and access to reliable data is critical.
Critics argue that withholding research results due to concerns over their potential misuse sets a dangerous precedent. Amy Tishelman, a clinical and research psychologist who was involved in the study, told The New York Times that while she understands the fear of data being “weaponized,” the results should be made public. “No change isn’t necessarily a negative finding—there could be a preventative aspect to it,” she said, emphasizing the need for further investigation.
Erica Anderson, a clinical psychologist and expert on transgender youth, expressed her dismay, calling the decision “shocking” and “disturbing.” She argued that it is the responsibility of researchers to share their findings, regardless of the potential backlash, noting that “it’s not her prerogative to decide based on the results that she will or won’t publish them.”
Olson-Kennedy’s decision is rooted in concerns about how the study’s results could be used in legal battles against gender-affirming care for minors. She noted that critics might leverage the findings in court cases to challenge the use of puberty blockers, especially as more than 20 states have passed bans or restrictions on such treatments in recent years. Olson-Kennedy emphasized the need for a careful and thorough analysis before releasing the study, saying, “It has to be exactly on point, clear and concise. And that takes time.”
This delay has drawn criticism from those who believe that research should be released without bias, with the aim of fostering informed debate. Opponents of withholding the data argue that the results could contribute to a more nuanced understanding of the effects of puberty blockers and help inform future medical decisions.
The controversy around the study comes amid a broader international reevaluation of puberty blockers and gender-affirming care. England’s National Health Service (NHS) recently limited the use of puberty blockers for minors, following a review that found the evidence for their benefits was limited. Similarly, Finland’s leading pediatric gender medicine expert, Dr. Riittakerttu Kaltiala, has advised caution, stating that a majority of gender-questioning children eventually come to accept their bodies without the need for medical intervention.
In contrast, the 2011 Dutch study, which has served as a cornerstone for advocating puberty blockers, found that the treatment led to improved mental health and reduced emotional distress in transgender youth. The differing outcomes between the Dutch research and Olson-Kennedy’s study underscore the complexities of this evolving field.
With the scientific and medical community divided over the best approach to treating gender dysphoria in children, many stress the need for open access to research findings. Transparency in studies like Olson-Kennedy’s is seen as crucial for developing an evidence-based understanding of how best to support transgender youth. “We’re craving information about these medical treatments for gender-questioning youth,” Anderson said, adding that releasing such data helps ensure that the care provided is rooted in the best available science.
While Olson-Kennedy’s concerns about the potential misuse of the study’s findings are understandable, critics argue that the decision to withhold the data undermines the values of scientific integrity and public trust. As debates about gender-affirming care continue to unfold across the country, the need for clarity, honesty, and evidence has never been more pressing.
Big Pharma
Federal Watchdog Confirms FDA Mishandled 2022 Infant Formula Crisis
Published
2 years agoon
June 18, 2024
An audit conducted by the inspector general for the Department of Health and Human Services (HHS) has confirmed that the Food and Drug Administration (FDA) mishandled the 2022 infant formula crisis, exacerbating the situation, according to a House GOP panel chairperson.
Audit Findings and Congressional Response
Rep. Lisa McClain, chairwoman of the Food and Drug Subcommittee on Health Care and Financial Services, announced on Monday that the long-awaited audit substantiates findings by the House Oversight Committee. “The 2022 nationwide infant formula crisis was exacerbated by dysfunction and delay within the FDA,” McClain stated.
The audit highlights critical failures within the FDA, including its failure to heed whistleblower warnings, conduct adequate inspections, and respond swiftly to the unfolding crisis. These shortcomings, according to McClain, had “serious implications” for the availability of infant formula across the nation.
Details from the Audit
The inspector general’s audit provides a detailed examination of the FDA’s actions and inactions during the crisis. Key points from the audit include:
- Ignored Whistleblower Warnings: The FDA reportedly failed to act on early warnings from whistleblowers within the industry who flagged potential issues with formula production and safety.
- Inadequate Inspections: The audit found that the FDA did not conduct timely and thorough inspections of formula manufacturing facilities, which could have identified and mitigated risks earlier.
- Slow Responsiveness: The FDA’s delayed response to emerging problems contributed significantly to the crisis, affecting the supply chain and availability of infant formula nationwide.
Implications and Accountability
Rep. McClain emphasized the serious implications of the FDA’s mishandling, which resulted in widespread shortages and panic among parents. She noted that multiple former FDA officials had testified before the subcommittee, corroborating the audit’s findings and shedding light on the agency’s internal failures.
“We are going to continue to use the tool of oversight to ensure that the FDA implements the recommendations in this report,” McClain said. She stressed the importance of the FDA making necessary adjustments to their policies and procedures and holding affiliated parties accountable for their roles in the crisis.
Future Actions
The subcommittee, led by McClain, plans to closely monitor the FDA’s implementation of the audit’s recommendations. The goal is to ensure that the agency adopts more effective practices and policies to prevent similar crises in the future. “This audit is a crucial step in understanding what went wrong and how we can fix it,” McClain added.
The findings of the HHS inspector general’s audit serve as a critical reminder of the need for vigilant oversight and accountability within federal agencies, particularly those responsible for public health and safety. The House GOP panel, under McClain’s leadership, aims to push for reforms that will restore confidence in the FDA’s ability to manage and mitigate such crises effectively.
The full report by the inspector general is expected to be a cornerstone for future legislative and administrative actions aimed at bolstering the FDA’s operational efficiency and responsiveness.

Tim Walz asked Minnesota assassin to kill Senator Klobuchar so he could take seat, FBI letter claims

ChatGPT use linked to cognitive decline, MIT research finds

Kamala Harris Allegedly Covered Up Biden’s Mental Decline, Democratic Source Says

Biden Administration Lost Track of Billions in Seized Crypto

Tim Walz asked Minnesota assassin to kill Senator Klobuchar so he could take seat, FBI letter claims


You must be logged in to post a comment Login